COPPER EXTRACTION: EXTRACTION OF COPPER FROM COPPER PYRITE
Principle
Copper is extracted mainly from its copper pyrite ore by means of the self-reduction process. Following are the steps involved in the extraction:
1. Crushing: The big lumps of copper pyrite ore is crushed in two smaller pieces by using a Jaw crusher.
2. Pulverzition: The crushed ore is further crushed and changed into powder form by using a Stamp mill.
3. Concentration: The concentration is done by Froth Floatation Method. In this method, the mixture of water and pine oil is taken on the vessel in which the powder ore from the pulverization process is mixed. The sulphide ore is wetted by pine oil and the impurities are wetted by water and settle down at the bottom of the vessel.
4. Roasting: It is done in the Reverberatory furnace by the supply of hot air. In this process, the moisture is removed and the volatile impurities are removed as an oxide.
In this process, the sulphide ore is converted into oxide ore by making it partially oxidized.
2CuFeS2 + O2 ⇾ Cu2S + 2FeS + SO2
2CuFeS2 + 4O2 ⇾ Cu2S + 3SO2 + 2FeO
5. Smelting: It is done in the Blast furnace. In this process, the ore is mixed with sand and coke. The silica reacts with iron oxide (FeO) to form a slag which is removed continuously. The two molten layers are formed. One is of slag which floats on the surface and another is the mixture of Cu2s and Fes which is called copper matte.
2FeS + 3O2 ⇾ 2FeO + 2SO2
FeO + SiO2 ⇾ FeSiO3 (slag)
 |
| [ Source: QsStudy ] |
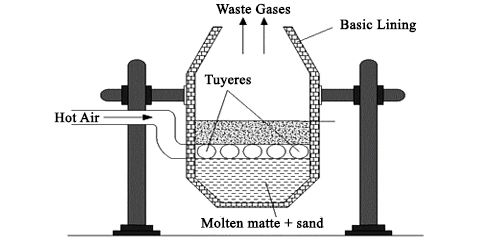
6. Bessemerization: The copper matte is transferred into the Bessemer converter and mixed with silica in which the remaining iron is removed in the form. The copper sulphide is changed into a copper oxide which again combines with copper sulphide to form copper.
2FeS + 3O2 ⇾ 2FeO + 2SO2
FeO + SiO2 ⇾ FeSiO3 (slag)
When the molten copper is cooled, the So2 gas escapes out forming the blisters on the surface which is called Blister Copper.
 |
| [ Source: QsStudy ] |
2Cu2S + 3O2 ⇾ 2Cu2O + 2SO2
Cu2S + 2Cu2O ⇾ 6Cu + SO2
PURIFICATION OF COPPER
1. Polling: In this method, the molten copper is stirred by using raw wood. The hydro-carbon present in the wood reduces the copper oxide into copper.
2. Electro Refining: The impure copper plate is taken as anode and pure copper plate as a cathode. CuSo4 is used as an electrolyte. When the electricity is passed, anodes dissolve and thin slabs of Cu-cathodes become converted to thick slabs of pure Cu. Thus, pure copper is obtained at the cathodes.
CuSO4 + 2Cu2O ⇾ Cu++ + SO42--
The pure copper is collected at the cathode and the impurities remain at the anode.
Thank You! for reading this Extraction of Copper: Extraction of Copper from Copper Pyrite Ore


Comments
Post a Comment
Please, do not enter any spam links in the comment box.